About the most exciting thing one can say about riboflavin is that if your take too much of it, your urine will take on a bright yellow/orange tint. Other than that, riboflavin is present in a lot of foods, more so in animal-sourced than plant-sourced, and that in the United States, deficiency is rare. Circa 1941, analysis of wheat flour confirmed that the process of making white flour destroyed approximately 85 percent of riboflavin so in the United States a fortification program was begun that added riboflavin – and other B vitamins – to white flour. As of 2021, 56 countries require food fortification of wheat flour or corn flour. An additional 16 countries have a voluntary fortification program. For example, the government of India recommends 4.0 mg/kg for "maida" (white flour) and for "atta" (whole wheat) flour.
As to what riboflavin does, it is a precursor to the creation of two coenzymes. Enzymes are proteins – strings of amino acids coded for by DNA and manufactured by RNA. In humans, there are some 70-80 enzymes that need the riboflavin coenzymes to function. Riboflavin is recycled in these processes, so the dietary amount needed to replace what is lost to daily urine output is remarkably small – on the order of 1.5 milligrams per day. One chicken egg provides about 0.2 mg, a glass of milk about 0.4 mg. There are no known adverse effects from consuming large quantities because absorption has an upper limit, and what is absorbed in excess of needs is quickly excreted in urine.
No known health benefits can be attributed to consuming amounts in excess of what is needed to prevent deficiency. In many countries, a chemical compound is either naturally occurring in food or a drug, with strict regulations concerning the latter. However, in the United States, passage of the Dietary Supplement Health and Education Act of 1994 (DSHEA) allowed for the existence of a middle category, dietary supplements, that could be manufactured and marketed as long as there is clear evidence that these products are safe and some scientific evidence in support of a purported health benefit.
Basically, in the U.S., this means that a company cannot sell a drug until the Food and Drug Administration (FDA) approves, but can sell a dietary supplement until the FDA or Federal Trade Commission says to stop.
The FDA restricts label health claims to specific wording referred to as a Structure:Function claim. S:F wording is limited to phrasing such as “Supports…” or “Promotes…” or “Helps…” One example from a riboflavin product label reads “Promotes Healthier Blood, Nervous System and Helps Boost Energy and Metabolism.” Is this manner, riboflavin is sold in doses as high at 400 mg despite the fact that deficiency is avoided by consuming 1.5 mg/day. Dietary supplement labels are also required to include a black-line outlined statement concerning the S:F claims: “These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.” Despite these restrictions, the supplement business in the United States exceeds $35 billion per year. The products range from the mundane (multi-vitamin/mineral complex) to the exotic (powdered deer antler velvet).
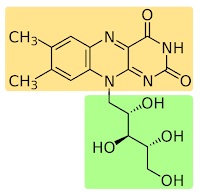 |
| Riboflavin chemical structure, with the ribose portion in green and the flacvin in yellow. |
In 1935, researchers reported that a yellow-fluorescing compound extracted from yeast restored normal growth when fed to rats. The compound was briefly referred to as "Vitamin G" before a consensus was reached for “riboflavin” and also “Vitamin B2.” Richard Kuhn was awarded the Nobel Prize in Chemistry in 1938 for his work on riblflavin. A year later it was confirmed that riboflavin is essential for human health through a clinical trial in which women fed a diet low in riboflavin developed stomatitis and other signs of deficiency, all of which were reversed when treated with synthetic riboflavin. As confirmation, the symptoms returned when the supplements were stopped.
Mark’s day job, until he retired, was providing expert scientist advice to dietary supplement, sports nutrition and medical nutrition companies wanting to make health claims.
No comments:
Post a Comment